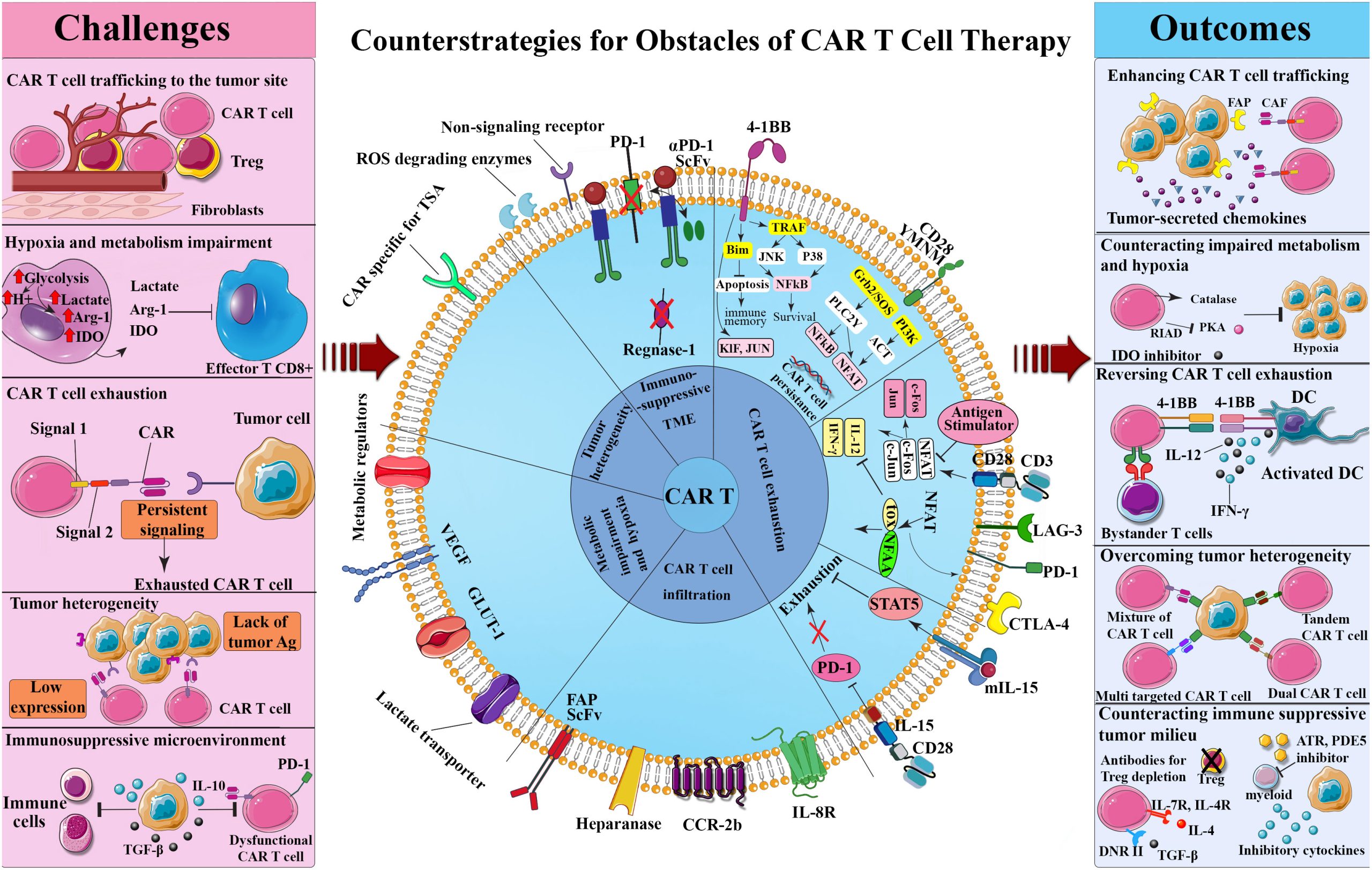
The FDA is concerned about the possibility of secondary malignancies in cancer patients treated with chimeric antigen receptor T-cell (CAR-T cell) immunotherapy. The FDA sent numerous manufacturers notification letters to put a “boxed warning” on these treatments’ prescription instructions. BCMA- and CD19-directed genetically engineered autologous T-cell immunotherapies can cause T-cell malignancies, some of which cause hospitalization and death.
CAR (chimeric antigen receptor) T cell, illustration. Photo from Google
FDA Warns of CAR-T Therapy Secondary Cancer Risk
As of the last update, the FDA has received 25 reports of CAR-T cell immunotherapies-related T-cell malignancy. Despite warnings, the FDA says some medicines’ benefits outweigh the dangers. The government advises monitoring people getting these treatments for new cancers throughout their lives.
CAR-T therapies change a patient’s T-cells in a lab to attack and infuse cancer cells. Abecma, Breyanzi, Carvykti, Kymriah, Tecartus, and Yescarta are FDA-approved CAR-T cell immunotherapies. These medicines were approved with 15-year follow-ups to determine safety and secondary cancer risk.
Previously a “class warning” in prescribing instructions, secondary malignancies are now a “boxed warning.” Due to the elevated risk of immune system malignancies, the FDA has advised manufacturers to revise package information and Medication Guides.
READ ALSO: Eyes Wide Open: 25p Energy Drinks Fuel Insomnia Surge, Scientists Issue Urgent Warning
FDA Advocates Lifelong Monitoring Amid Secondary Cancer Concerns
According to the FDA, CAR-T patients and clinical trial participants should be monitored for new cancers for life. Healthcare practitioners should report post-treatment malignancies to the manufacturer and follow sample collection instructions.
The FDA’s CAR-T therapy investigation began in November to assess secondary cancer risk. Despite the documented cases, the FDA maintains that CAR-T therapy converts relapsed or resistant blood cancer patients, making the risk-benefit balance highly advantageous. Oncologists say CAR-T therapy’s advantages outweigh their risks despite the risk of secondary malignancies. Patients who need these medicines generally die from their cancers, but CAR-T cell therapies have increased response rates and durability.
Doctors and patients may make educated treatment decisions by adding risk information to safety labeling. Despite the FDA’s warnings, healthcare providers advocate CAR-T therapy, noting their adverse effects but underlining their proven efficacy in treating certain tumors.
READ ALSO: Discovering How Milk Can Boost Your Health