Morayo Ogunbayo | (TNS) The Atlanta Journal-Constitution
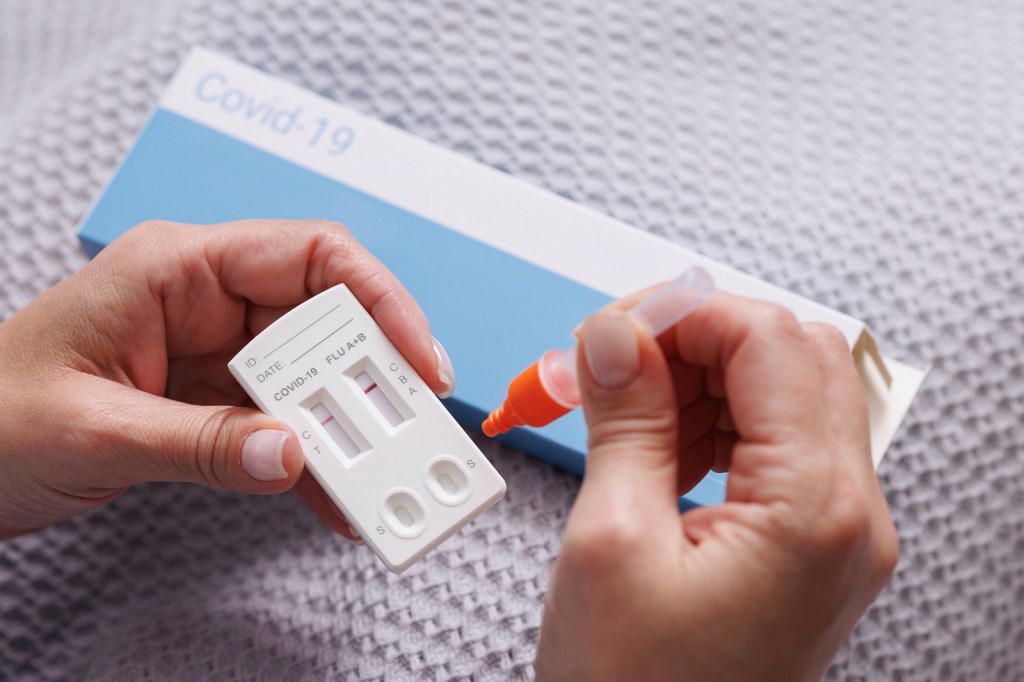
You probably have a cabinet full of rapid COVID-19 tests you’ve accumulated. The expiration dates have come closer and closer, with some tests even reaching them.
The Food and Drug Administration, however, has said those expiration dates are subject to change, providing a list of the tests that have had their dates extended.
COVID-19 rapid antigen tests allow people to check for SARS-CoV-2 infections without help from professionals. They provide positive or negative results for the virus, typically within 15 minutes.
Rapid COVID tests list their shelf life — how long the test should work as expected — and expiration date — the date through which the test is expected to perform accurately — on the box. According to the FDA, expiration dates can be extended when the manufacturer provides data showing the shelf life is longer than originally expected.
Finding the shelf life, called stability testing, often takes a long time for test manufacturers, with the FDA opting to give them a shelf life of four to six months instead of waiting. After the test maker finds the true results of stability testing, they will contact the FDA with their new date.
The FDA has extended the expiration date for hundreds of tests, available to search here.